Introduction to Superheat: How To Calculate Superheat Formula
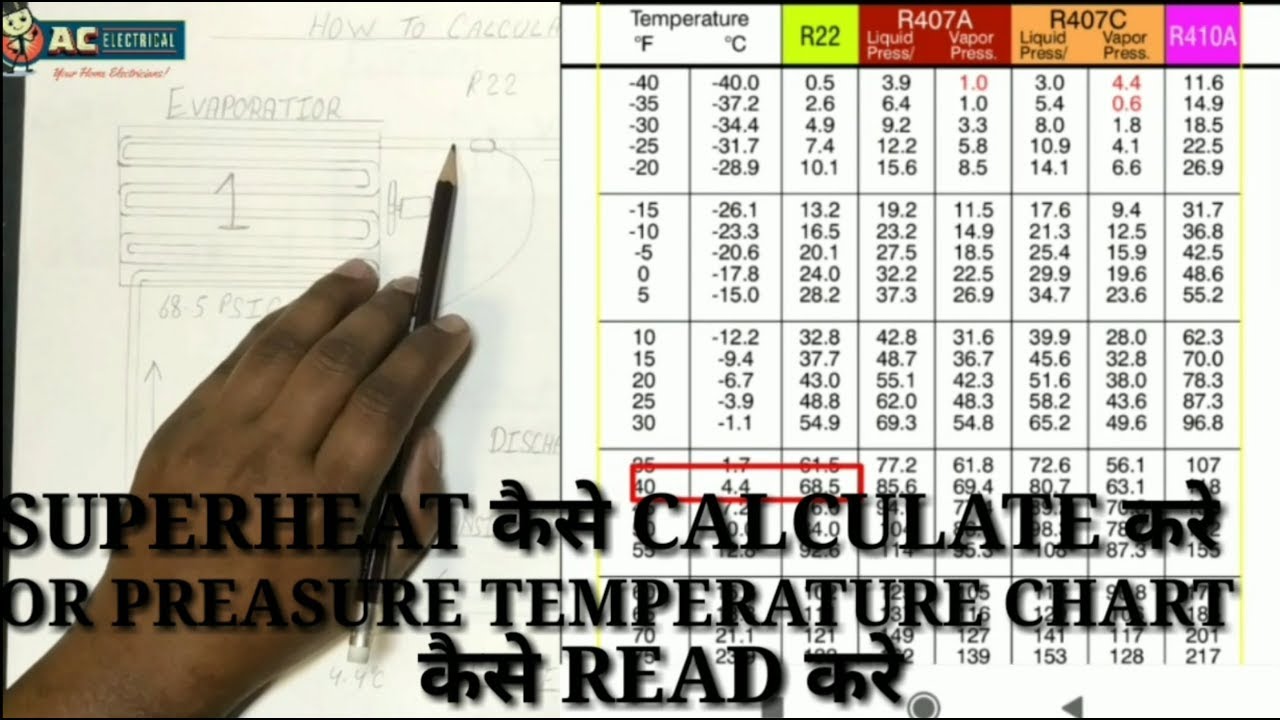
Superheat refers to the state of a substance, typically steam, where its temperature exceeds the saturation temperature corresponding to its pressure. This concept is crucial in thermodynamics and various engineering applications, particularly in power generation and industrial processes. Understanding superheat allows engineers to optimize system performance and efficiency.
The concept of superheat is vital in steam power plants, where controlling and measuring superheat is critical for maximizing power output and minimizing potential issues like condensation or erosion. Superheat is also essential in other applications, such as industrial processes involving steam as a working fluid. Precise control of superheat is crucial for maintaining the desired process parameters and preventing unexpected operational problems.
Definition of Superheat
Superheat is the amount by which the temperature of a substance, typically steam, exceeds its saturation temperature at a given pressure. This difference in temperature is crucial for understanding the thermodynamic state of the substance and its behavior in various applications.
Concept of Superheat in Steam and Thermodynamics
Superheated steam is steam that has been heated beyond its saturation temperature at a specific pressure. This additional heat energy increases the kinetic energy of the steam molecules, contributing to higher enthalpy and internal energy. Understanding superheat is fundamental to calculating the properties of steam in thermodynamic cycles, including power generation systems.
Importance of Understanding Superheat
Precise control of superheat is vital in numerous applications, including:
- Steam Power Plants: Optimizing power output and efficiency is directly related to precise superheat control. Maintaining the right superheat prevents issues like condensation and erosion in turbines, leading to increased lifespan and reduced maintenance.
- Industrial Processes: Many industrial processes use steam as a working fluid. Precise superheat control is crucial for achieving the desired process parameters and preventing problems like premature equipment failure or unexpected reactions.
- Heating and Cooling Systems: Superheat is important in systems involving steam as a heat transfer medium. Precise control of superheat prevents condensation and ensures efficient heat transfer, leading to better energy efficiency.
Key Parameters Affecting Superheat
Several key parameters influence the degree of superheat:
- Pressure: The pressure of the steam significantly impacts the saturation temperature. Higher pressure corresponds to a higher saturation temperature, thus affecting the degree of superheat required to achieve a specific temperature.
- Heat Input: The amount of heat added to the steam determines the degree of superheat. More heat input results in greater superheat.
- Steam Properties: The inherent properties of the steam, such as initial temperature and moisture content, influence the amount of superheat required to achieve the desired conditions.
Simple Analogy for Superheat
Imagine boiling water on a stove. The water boils at 100°C (at sea level). If you continue to heat the water, its temperature rises above 100°C, entering a superheated state. Similarly, superheated steam is steam that’s been heated beyond its boiling point (saturation temperature) at a given pressure.
Understanding the Formula
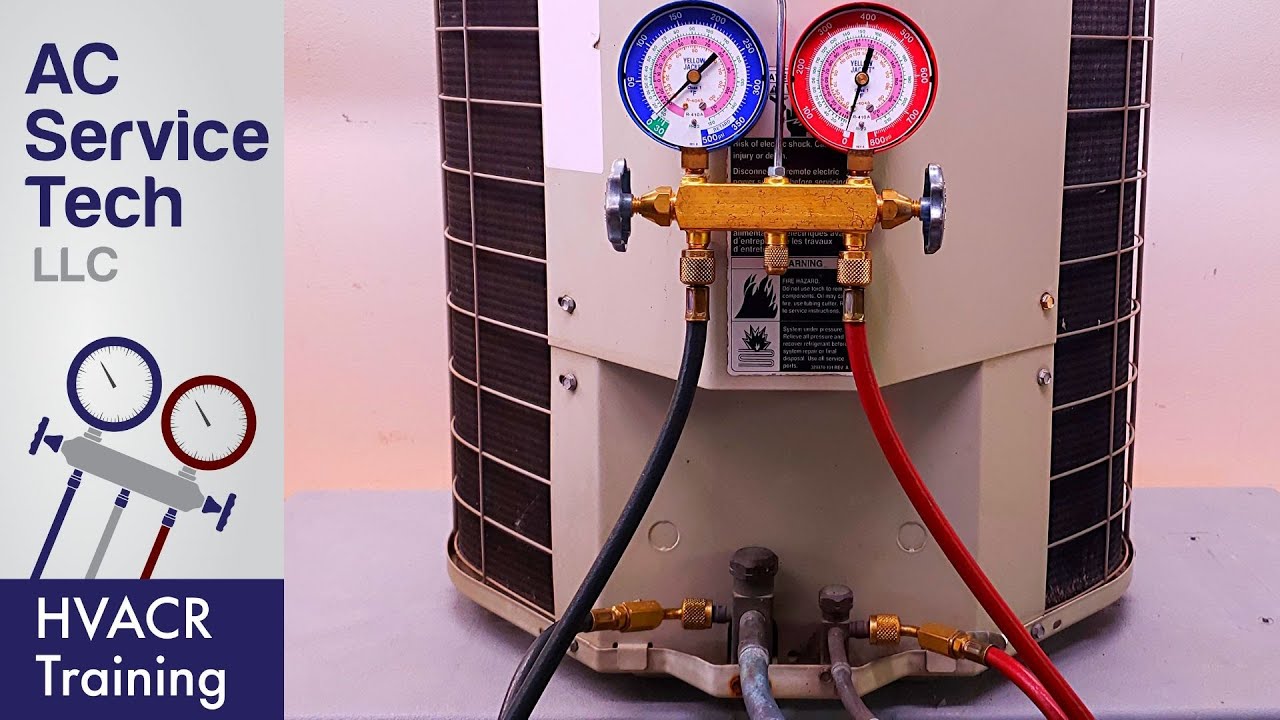
The superheat formula, crucial for thermodynamic analysis, defines the degree of superheat in a substance. Understanding its variables, units, and significance allows for accurate calculations and interpretation of thermodynamic processes. This section delves into the specifics of the formula, encompassing various forms and calculation methods.
The superheat formula quantifies the temperature difference between the actual temperature of a substance and its saturation temperature at a given pressure. This difference is essential in various engineering applications, from power plant design to refrigeration systems. A deep understanding of the formula empowers engineers to optimize processes and ensure efficient operation.
Variables in the Superheat Formula
The core of the superheat formula involves several variables, each playing a specific role in the calculation. Proper understanding of these variables and their associated units is paramount for accurate results.
- Temperature (T): Represents the actual temperature of the substance. Its value determines the level of superheat. Units commonly used are degrees Celsius (°C) or Kelvin (K).
- Saturation Temperature (Tsat): Corresponds to the temperature at which a substance changes phase (e.g., from liquid to vapor) at a particular pressure. This is a critical reference point for determining the degree of superheat. Units are the same as temperature (T).
- Pressure (P): Refers to the pressure exerted on the substance. Pressure directly impacts the saturation temperature and, consequently, the superheat value. Units are typically in Pascals (Pa), bars, or pounds per square inch (psi).
Significance of Variables
Each variable in the superheat formula holds significant importance. The interplay of these variables defines the state of the substance and its thermodynamic properties.
- Temperature (T): A direct measure of the substance’s thermal energy, it directly reflects the superheat level.
- Saturation Temperature (Tsat): Acts as a reference point for assessing the deviation from the saturation state, providing insight into the degree of superheat.
- Pressure (P): Impacts the saturation temperature. Changes in pressure directly affect the substance’s saturation state, influencing the superheat value.
Different Forms of the Superheat Formula
Several forms of the superheat formula exist, depending on the specific thermodynamic properties being considered. Each form can be useful for different scenarios.
- The simplest form typically involves calculating the difference between the temperature and the saturation temperature:
Superheat = T – Tsat
This directly reflects the amount of superheat present.
Methods for Calculating Superheat
Different approaches exist for calculating superheat, each with its own advantages and limitations. The most common method involves using thermodynamic property tables or software to determine the saturation temperature at the given pressure.
- Using Thermodynamic Tables: These tables provide a comprehensive database of thermodynamic properties for various substances, including saturation temperature at different pressures. This is a reliable method for obtaining accurate superheat values.
- Using Thermodynamic Software: Modern software packages offer powerful tools to calculate thermodynamic properties, including saturation temperatures, quickly and accurately. Software allows for complex calculations and is particularly beneficial for intricate systems.
Calculating Superheat

Calculating superheat involves determining the temperature difference between the actual temperature of a substance and its saturation temperature at a given pressure. This difference is crucial in various engineering applications, from power plant design to process control. Accurately calculating superheat ensures efficient operation and safety.
Understanding the specific methods for calculating superheat is essential for reliable estimations and analyses. This section will Artikel the common steps and illustrate the process with examples, highlighting the significance of precise input data.
Step-by-Step Procedure for Calculating Superheat
A common approach involves utilizing thermodynamic property tables or software. These resources provide data points for specific substances at various pressures and temperatures, including saturation properties. To calculate superheat, you need to know the pressure and the temperature of the substance.
- Identify the Substance and its State: Determine the substance undergoing superheating and its initial state (pressure and temperature).
- Locate Saturation Properties: Using thermodynamic property tables or software, find the saturation temperature corresponding to the known pressure. This saturation temperature is the temperature at which the substance changes phase (from liquid to vapor).
- Calculate the Superheat: Subtract the saturation temperature from the actual temperature of the substance. This difference is the superheat value.
- Units of Measurement: Ensure consistent units (e.g., degrees Celsius or Kelvin) throughout the calculation.
Common Calculation Methods
Different methods can be used for calculating superheat, each with its own advantages and limitations. Choosing the right method depends on the available data and the level of accuracy required.
| Method | Description |
|---|---|
| Thermodynamic Property Tables | These tables list tabulated values of properties (e.g., enthalpy, entropy, temperature) for various substances at different conditions. Using these tables requires careful interpolation between listed values. |
| Software Tools | Software packages provide efficient and accurate calculations of thermodynamic properties, including superheat. Software avoids manual interpolation and often handles complex calculations. |
| Empirical Correlations | These equations relate properties based on experimental data. They are suitable for substances for which detailed property tables are not readily available. |
Examples of Superheat Calculations
These examples demonstrate the calculation process using different initial conditions and desired outcomes.
- Example 1: Calculate the superheat of steam at a pressure of 100 kPa and a temperature of 120°C. Using steam tables, the saturation temperature at 100 kPa is approximately 99.63°C. Therefore, the superheat is 120°C – 99.63°C = 20.37°C.
- Example 2: Calculate the superheat of refrigerant R-134a at a pressure of 1.5 MPa and a temperature of 60°C. Using thermodynamic property tables, the saturation temperature at 1.5 MPa is approximately 30°C. The superheat is 60°C – 30°C = 30°C.
Implications of Errors in Input Data
Errors in the input data (pressure or temperature) directly impact the calculated superheat value. A small error in pressure or temperature can lead to a significant deviation in the superheat calculation. For instance, if the pressure is incorrectly measured, the corresponding saturation temperature will also be inaccurate, leading to an erroneous superheat value. Careful attention to measurement accuracy is critical for reliable results.
Practical Applications
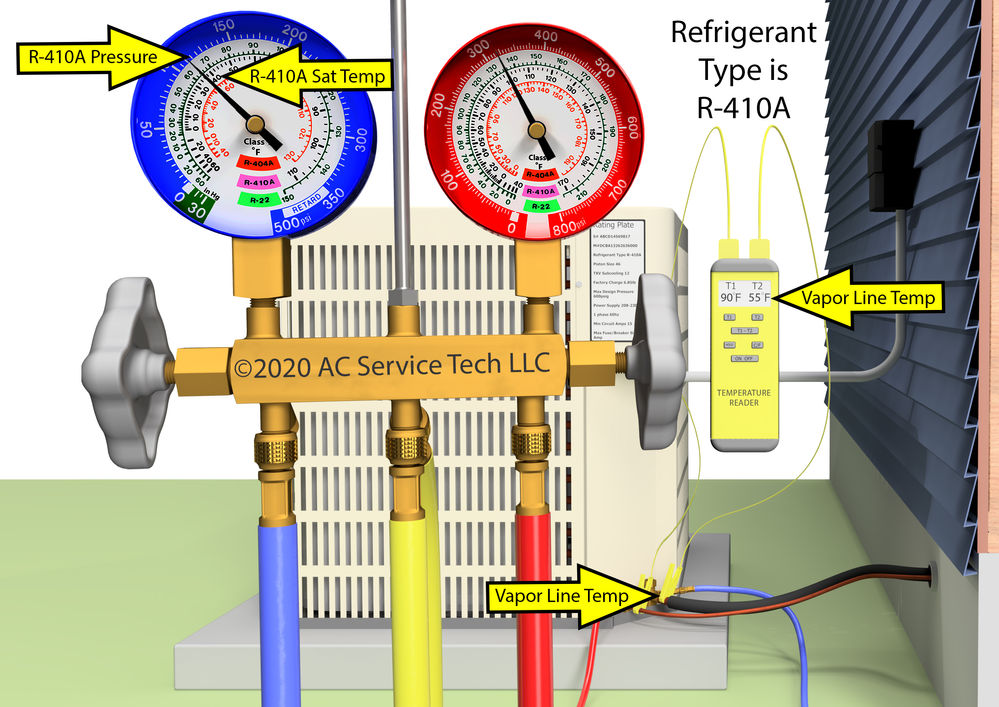
Superheat plays a crucial role in various engineering applications, particularly in thermal power generation and industrial processes. Understanding and controlling superheat is essential for maximizing efficiency and ensuring safe operation. This section explores the significance of superheat in different contexts.
The precise control of superheat is paramount in ensuring optimal performance and safety in systems utilizing high-temperature steam. Accurate superheat calculations are essential for engineers to design and operate these systems effectively.
Superheat in Power Plants
Superheat is a key element in modern power plants, particularly those using steam turbines. By increasing the temperature of the steam beyond its saturation point, superheat enhances the thermodynamic efficiency of the entire power generation process.
- Improved Turbine Efficiency: Superheated steam expands more efficiently within the turbine blades, leading to higher power output for a given input of fuel. This enhanced efficiency translates directly to lower operational costs and higher overall plant productivity.
- Reduced Thermal Stress: Superheated steam experiences less temperature fluctuations compared to saturated steam, which minimizes thermal stress on the turbine components. This extended lifespan and reduced maintenance costs are significant benefits.
- Enhanced Plant Output: The increased efficiency of the steam cycle, due to superheat, leads to higher overall plant output and lower emissions per unit of electricity produced. This aligns with the drive towards sustainable energy practices.
Superheat in Steam Turbines
Steam turbines rely heavily on superheated steam for optimal performance. The higher the temperature of the superheated steam, the more efficiently it expands within the turbine, leading to increased power generation.
- Power Output Enhancement: Superheated steam allows for a greater power output from the turbine, directly correlating with a higher efficiency in the power generation process. This is a key driver in maximizing power plant output.
- Reduced Moisture Content: Superheating reduces the moisture content in the steam, which is crucial for preventing erosion and damage to turbine blades. This is a vital safety consideration, minimizing downtime and maintenance costs.
- Improved Thermal Efficiency: Superheating enhances the thermal efficiency of the steam cycle, leading to greater overall power generation and lower energy consumption per unit of output. This is a critical factor in achieving sustainable power production.
Superheat in Industrial Processes
Superheated steam is utilized in a wide range of industrial processes, from heating and processing to sterilization and material treatment. The benefits of superheat extend beyond power generation.
- Enhanced Process Efficiency: Superheated steam provides a more consistent and intense heat source, leading to faster reaction rates and improved efficiency in various industrial processes. This is particularly relevant in chemical and metallurgical industries.
- Improved Material Properties: Superheated steam is used in processes involving material treatment, such as drying and sterilization. This leads to improved product quality and consistency.
- Increased Production Capacity: By improving process efficiency, superheat can contribute to a higher overall production capacity and reduced processing time. This is a significant factor in maintaining competitiveness in the industrial sector.
Critical Situations for Superheat Calculation
Accurate superheat calculations are critical in various situations, especially during plant start-up and shutdown, and when dealing with fluctuating operating conditions.
- Plant Start-up and Shutdown: Precise calculations are crucial during plant start-up to ensure that the steam temperature reaches the desired superheat level for optimal turbine operation. Similarly, accurate calculations are vital during shutdown to avoid potential equipment damage.
- Fluctuating Operating Conditions: When plant conditions change, such as varying steam pressure or flow rate, superheat calculations are needed to ensure continuous optimal performance. This is particularly important for maintaining the required superheat level to prevent turbine damage.
- Equipment Design and Maintenance: Superheat calculations are integral to the design and maintenance of steam-powered equipment. This includes ensuring safety and efficiency throughout the operational cycle.
Impact of Superheat on Efficiency
Superheat directly impacts the efficiency of various applications by influencing the thermodynamic properties of the steam.
- Increased Thermal Efficiency: By increasing the temperature of steam, the thermal efficiency of the entire cycle is enhanced, leading to better utilization of the input energy. This is evident in both power plants and industrial processes.
- Reduced Energy Consumption: Higher superheat levels translate to lower energy consumption per unit of output, which is a significant factor in cost-effectiveness and sustainability. This improvement in energy efficiency directly translates to savings.
- Improved System Performance: Superheat significantly enhances the performance of steam-powered equipment, making them more efficient and productive. This improvement is crucial for overall system optimization.
Factors Affecting Superheat

Superheat, the temperature difference between the actual temperature of a substance and its saturation temperature at a given pressure, is crucial in various engineering applications. Understanding the factors influencing superheat is vital for precise calculations and optimal system performance. These factors often interact, making a comprehensive analysis essential.
External Factors Influencing Superheat
External factors, such as pressure and temperature variations, significantly impact the superheat value. These factors are independent of the substance’s intrinsic properties.
- Pressure Variations: Pressure directly affects the saturation temperature of a substance. A rise in pressure typically results in a higher saturation temperature. Consequently, if the temperature of the substance remains constant, a higher pressure will decrease the superheat. Conversely, a drop in pressure leads to a lower saturation temperature, increasing the superheat value at the same temperature. This relationship is fundamental to superheat calculations.
- Temperature Variations: Temperature variations, independent of pressure, influence superheat. If the pressure remains constant, an increase in the substance’s temperature leads to an increase in superheat. This is because the difference between the substance’s temperature and its saturation temperature at the constant pressure grows.
Heat Input and System Design Impact
The amount of heat input and the design of the system significantly affect the superheat level. Careful consideration of these aspects is critical for achieving the desired superheat values.
- Heat Input: The amount of heat supplied to the substance directly impacts its temperature rise. More heat input generally leads to a greater temperature difference between the substance’s temperature and its saturation temperature, resulting in a higher superheat value. Factors like the rate of heat input and the system’s thermal efficiency also play a crucial role.
- System Design: System design elements like heat exchangers, pipes, and insulation significantly influence superheat. Proper insulation minimizes heat loss, ensuring a more accurate superheat calculation. The design of heat exchangers impacts the rate of heat transfer, which in turn affects the superheat achieved. A poorly designed system might lead to inaccurate superheat values.
Fluid Properties and Superheat Calculations
Fluid properties have a substantial impact on superheat calculations. The unique characteristics of each substance dictate how it responds to changes in temperature and pressure.
- Specific Heat: The specific heat of a substance indicates how much heat is required to raise its temperature by a unit degree. A substance with a high specific heat requires more heat input to achieve the same temperature increase compared to a substance with a low specific heat, which will influence superheat.
- Latent Heat: The latent heat of a substance quantifies the energy absorbed or released during a phase change. Substances with higher latent heat values can absorb significant heat without substantial temperature increases, which affects the superheat calculations during phase transitions.
- Thermal Conductivity: The thermal conductivity of a substance indicates how readily it transfers heat. High thermal conductivity substances facilitate faster heat transfer, influencing the rate at which superheat is achieved.
Material Impact on Superheat Calculations
The materials used in the system, such as pipes, vessels, and heat exchangers, can influence superheat calculations. Different materials have varying thermal properties that can affect the accuracy of superheat calculations.
- Thermal Conductivity of Materials: The thermal conductivity of the materials used in the system influences heat transfer. Materials with high thermal conductivity allow for faster heat transfer, potentially leading to higher superheat values in certain situations.
- Material Properties: Materials with high thermal expansion rates can affect the system’s geometry, potentially impacting heat transfer rates and thus superheat calculations. Materials with different specific heats can also influence the final superheat.
Troubleshooting and Error Analysis
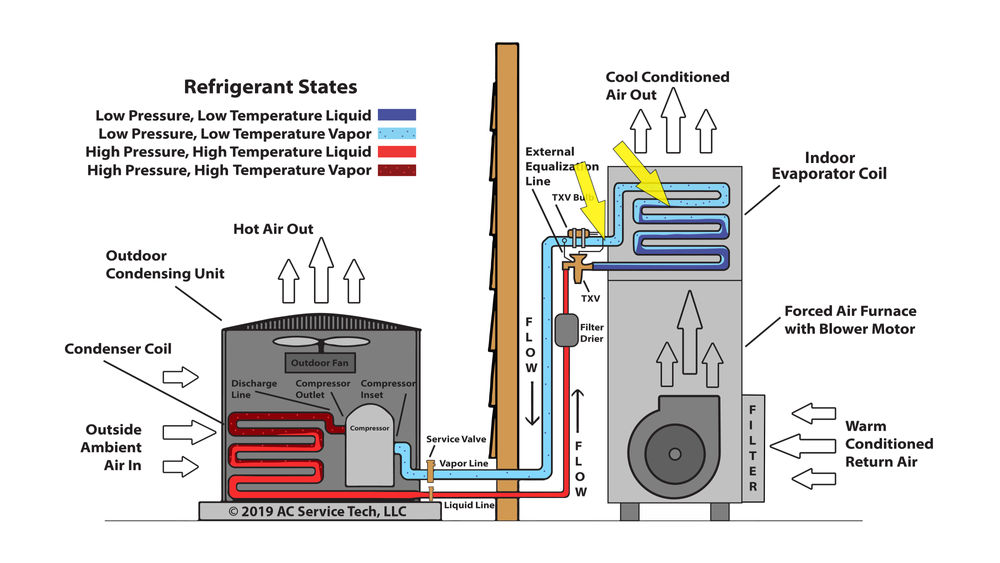
Accurately determining superheat is crucial in various engineering applications. However, errors in calculations can lead to significant discrepancies in the final results, potentially affecting system performance and safety. This section details potential pitfalls in superheat calculations and provides strategies for identifying and rectifying them.
Identifying and correcting errors in superheat calculations is vital to ensure accurate results and reliable system performance. A meticulous approach to data input, formula application, and result interpretation is paramount.
Potential Errors in Superheat Calculations
Several factors can contribute to inaccuracies in superheat calculations. These include incorrect input data, inappropriate selection of thermodynamic properties, or procedural errors during the calculation process. Input errors, such as misreading pressure gauges or temperature sensors, directly affect the calculated superheat value. Likewise, using the wrong thermodynamic property tables or charts for the specific substance or condition can lead to significant inaccuracies.
Methods to Identify and Rectify Errors
Careful review of the input data is the first step in error detection. Double-checking measured values against instrument calibrations and ensuring the correct units are used is critical. Verification of the thermodynamic properties is equally important. Cross-referencing data from multiple reliable sources and comparing results to established values can pinpoint inconsistencies.
Interpreting Results and Identifying Anomalies
Analyzing the calculated superheat value within the context of the system is essential. Deviations from expected values, especially large deviations, warrant further investigation. For example, if the calculated superheat is significantly higher or lower than anticipated, it might indicate a measurement error, a data input error, or an issue with the thermodynamic model being used. A comparison of the calculated superheat with established industry standards and best practices is a beneficial diagnostic tool.
Common Causes of Inaccuracies
Inaccurate measurements, using outdated or inappropriate thermodynamic property tables, and errors in the calculation procedure itself can contribute to inaccuracies in superheat calculations. Inconsistent or poorly maintained equipment used to measure temperature or pressure will also introduce errors in the data.
Troubleshooting Steps for Different Types of Errors
| Error Type | Troubleshooting Steps |
|---|---|
| Incorrect Input Data |
|
| Inappropriate Thermodynamic Properties |
|
| Calculation Procedure Errors |
|
Advanced Concepts (Optional)

Superheat calculations, while fundamental, can be further enriched by exploring advanced concepts. These supplementary areas provide deeper insights into the behavior of superheated steam and offer more sophisticated approaches for analysis and practical application. This section delves into advanced software, isentropic superheat, thermodynamic properties, measurement techniques, and limitations of calculations.
Advanced Software and Tools
Modern engineering software packages often incorporate sophisticated algorithms and extensive thermodynamic property databases. These tools streamline superheat calculations and provide more precise results than manual calculations, particularly for complex systems. For instance, commercial software packages can model multi-component mixtures, incorporate heat transfer effects, and accommodate variations in pressure and temperature more accurately. This automated approach can significantly reduce errors and allow for detailed analyses of real-world scenarios.
Isentropic Superheat
Isentropic superheat refers to the increase in temperature of a steam stream when it is compressed adiabatically (no heat transfer) and reversibly. This theoretical process provides a benchmark for evaluating the actual superheat achieved in real-world applications. A comparison between actual and isentropic superheat reveals the irreversibilities and inefficiencies inherent in the system. This is critical in evaluating the performance of turbines and other steam-powered equipment.
Thermodynamic Properties of Superheated Steam
Superheated steam exhibits a complex relationship between its pressure, temperature, enthalpy, and entropy. These properties are crucial for designing and analyzing steam-powered systems. Accurate determination of these properties is vital for reliable predictions of system performance. Changes in these properties are significantly influenced by the specific heat capacity, which varies with temperature. Tables and charts provide essential data for these thermodynamic properties, aiding in design calculations and system analysis.
Measuring Superheat
Several techniques are used to measure superheat, each with its advantages and limitations. Common methods include thermocouples, resistance temperature detectors (RTDs), and radiation pyrometers. Thermocouples, for example, are commonly used for direct temperature measurement, providing fast and relatively accurate readings. The choice of technique depends on the specific application and desired accuracy. For instance, radiation pyrometers might be more suitable for remote or high-temperature measurements.
Limitations of Superheat Calculations
Superheat calculations, like any engineering analysis, are subject to limitations. These limitations stem from factors such as the accuracy of input data, the assumptions made in the calculations, and the inherent complexity of the thermodynamic processes involved. For example, inaccuracies in pressure or temperature measurements can directly impact the calculated superheat value. Additionally, neglecting factors like heat transfer or friction losses can lead to significant errors in the prediction of superheat. Recognizing these limitations is crucial for ensuring the validity and applicability of the results.
Examples and Case Studies

Superheat calculations are crucial in various engineering disciplines, from power generation to chemical processing. Understanding how to apply these calculations correctly ensures efficient operation and avoids potential hazards. This section provides practical examples and case studies illustrating the importance of accurate superheat determination.
Illustrative Examples
This section presents a range of examples demonstrating superheat calculations for different substances. Accurate superheat calculations are essential for optimal system performance and safety.
| Substance | Initial Temperature (°C) | Initial Pressure (kPa) | Saturation Temperature (°C) | Superheat (°C) |
|---|---|---|---|---|
| Water | 120 | 100 | 100 | 20 |
| Steam | 250 | 1500 | 170 | 80 |
| Refrigerant R134a | -10 | 100 | -26 | 16 |
These examples showcase the diversity of substances requiring superheat calculations. Accurate determination of superheat is vital for ensuring proper system operation and avoiding potential issues.
Case Studies in Power Generation
Superheat plays a significant role in power plant efficiency. A case study examining the impact of inadequate superheat in a steam turbine illustrates the importance of precise calculations. A power plant experienced reduced efficiency due to insufficient superheat in its steam turbine, leading to operational losses. Improved superheat calculation procedures were implemented, resulting in a notable increase in plant efficiency and a reduction in operating costs. Such scenarios highlight the need for careful superheat determination in power generation systems.
Optimal Superheat Levels, How to calculate superheat formula
Determining the optimal superheat level depends on the specific application. Factors like desired process efficiency and equipment limitations influence the optimal superheat value. For instance, in a steam turbine, higher superheat can improve thermal efficiency, but excessive superheat can cause thermal stress on turbine components. Careful consideration of these factors is necessary to achieve optimal performance. Calculations must account for the specific characteristics of the working fluid and the equipment.
“Optimal superheat levels balance efficiency gains with material limitations, ensuring the longevity and efficiency of the system.”
Calculating Superheat for Different Substances
Calculating superheat involves determining the temperature difference between the actual temperature and the saturation temperature at a given pressure. This calculation is critical in various industrial processes, ensuring safe and efficient operation. The formula for calculating superheat is:
Superheat = Actual Temperature – Saturation Temperature
Using the appropriate thermodynamic tables or software for the specific substance allows for precise calculation.
Common Queries
How to calculate superheat formula – What are the common units of measurement used in superheat calculations?
Common units include degrees Celsius or Kelvin for temperature, Pascals or pounds per square inch for pressure, and specific enthalpy values often expressed in kilojoules per kilogram.
How does pressure variation affect superheat calculations?
Pressure changes influence the amount of superheat, as pressure and temperature are linked in thermodynamic processes. Increased pressure often leads to a higher superheat value at a given temperature.
What are the potential errors in superheat calculations, and how can they be identified?
Errors can arise from inaccurate input data, incorrect unit conversions, or flawed application of the chosen calculation method. Careful review of input data, consistent use of units, and comparison with known values can help identify potential errors.